Fresh off the press and open access (click the title):
Distinguishing between nociceptive and neuropathic components in chronic low back pain using behavioural evaluation and sensory examination
“Background: Diagnosis of chronic low back pain (CLBP) is traditionally predicated on identifying underlying pathological or anatomical causes, with treatment outcomes modest at best. Alternately, it is suggested that identification of underlying pain mechanisms with treatments targeted towards specific pain phenotypes may yield more success. Differentiation between nociceptive and neuropathic components of CLBP is problematic; evidence suggests that clinicians fail to identify a significant neuropathic component in many CLBP patients. The painDETECT questionnaire (PDQ) was specifically developed to identify occult but significant neuropathic components in individuals thought to have predominantly nociceptive pain…
…We hypothesised that patients with CLBP would exhibit poorer quality of life, greater psychological distress and also exhibit different sensory examination profiles compared to controls. In addition, we hypothesised that CLBP patients identified by PDQ as having a significant neuropathic component to their pain (irrespective of lesion or disease status) would complain of greater pain, poorer quality of life, greater psychological distress and also exhibit different sensory examination profiles compared to Group 1 patients.
Our data supported these hypotheses; group profiles reflect pain phenotypes identified by the PDQ. However, it may be suggested that group differences may be determined by other factors. For instance, psychological profiles and pain intensity differed in each group significantly. However, scores on the PDQ are not determined by any sort of psychological enquiry, nor are there any questions that assess pain intensity, so CLBP categorisation by PDQ is independent of these parameters. We believe, therefore, that these group differences reflect underlying pain mechanisms identified by the PDQ and are consistent with the concept of neuropathic signs and symptoms in LBP being a consequence of maladaptive plasticity of the nervous system and not solely a consequence of a lesion or disease process” (emphasis added)
It’s a really good paper – one of those rare ones that does some theoretical heavy lifting and conceptual hard work but is easy to read and immediately clinically relevant. While subgrouping back pain is a somewhat controversial topic, these findings suggest that the painDETECT questionnaire is quick, reliable, and could easily be included in routine low back pain assessment.
The notion of a pain phenotype doesn’t get mentioned much – but it’s worth reflecting on. The definition for phenotype; “the set of observable characteristics of an individual resulting from the interaction of its genotype with the environment” lends a nice embodied, embedded and enactive aspect when combined with pain. Perhaps for another post
Here’s the aforementioned PDQ
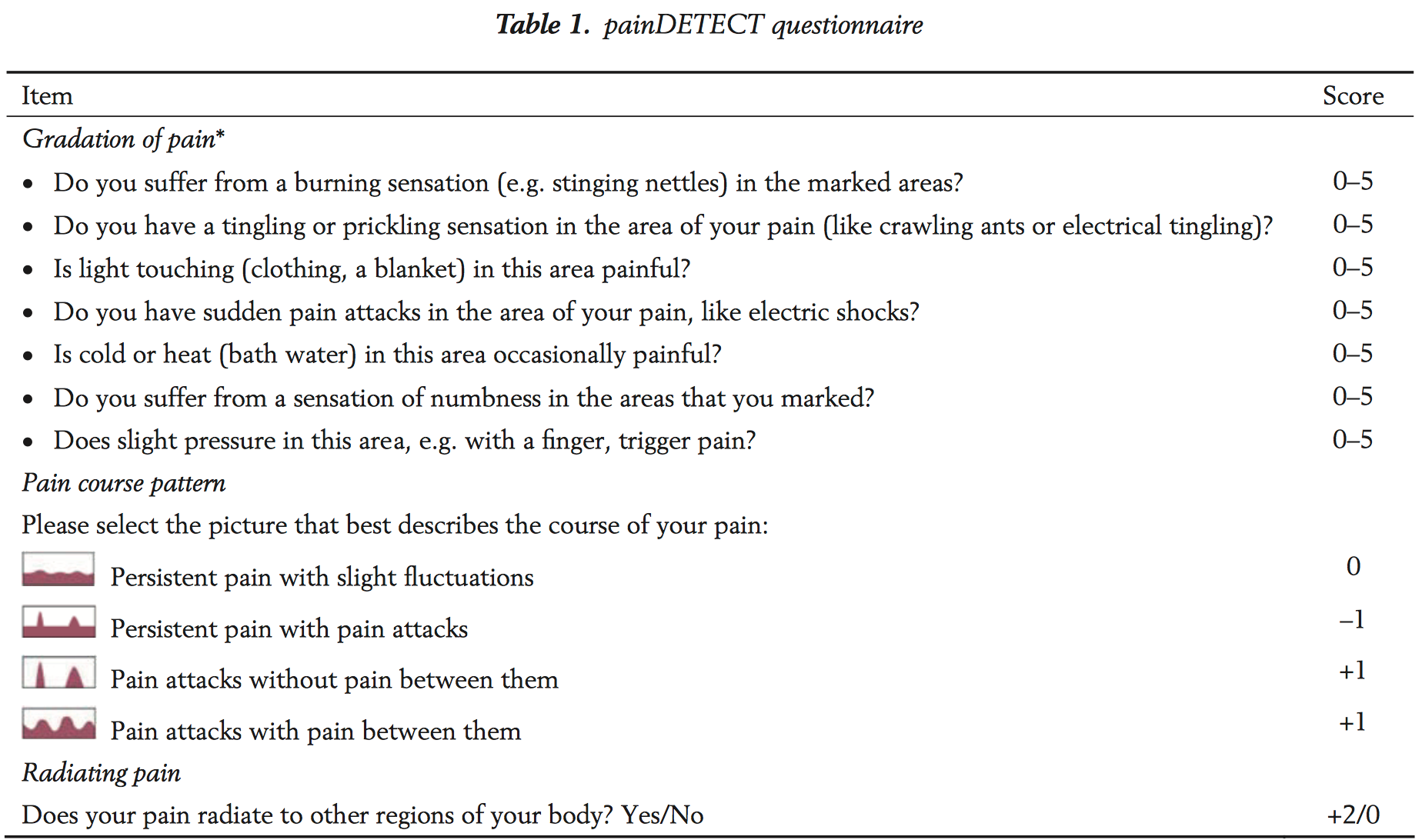
The development and validation of the PDQ is documented by Freynhagen et al (2006) – and there’s more open access goodness here if you want to read the whole thing (which you should, of course).
Outstanding questions
The original paper does leave a few questions outstanding – if there is no “demonstrable lesion or disease of the somatosensory nervous system” (the IASP guideline requirement to arrive at a neuropathic pain classification) what’s going on in those in Group 2? The authors provide a hint “a consequence of maladaptive plasticity of the nervous system and not solely a consequence of a lesion or disease process” but there’s a much bigger story there. A little birdie has told me that there’s more to come in a further paper reporting on the neuroimaging study (Study 2).
-Tim Cocks
Adelaide, 26-28 May EP + GMI
Wollongong, 14-16 July EP + GMI
Darwin, 4-6 August EP + GMI
Brisbane, 25-27 August EP + GMI
Newcastle, 8-10 September EP + GMI


Maladaptive plasticity of the nervous system? I cannot follow the reasoning behind this strange suggestion. Can someone please explain it to me?
The very interesting paper by Spahr et al.(2017) pushed me into some sort of reflections. The fact that patients with chronic pain suffer from different mechanisms is highly relevant. As the authors stated, nociceptive and neuropathic-like pain pose different labels and probably different treatment management programs. This is undoubtful, but do we can distinguish patients based on simple questionnaire measure? Or maybe more rigorous quantitative (QTS) approach should be employed? The interesting finding from the Spahr’s results is that patients from both groups (dominant nociceptive versus neuropathic) did differ in terms of two-point discrimination threshold. According to some authors, TPD is a measure of maladaptive plasticity in the CNS what is conflicting if one consider some neuroimaging data showing that maladaptive plasticity occurs ONLY in neuropathic-like pain. So… how to explain TPD changes in patients with nociceptive chronic low back pain? 🙂
Once again I ask for an explanation of “maladaptive plasticity”. What does it mean? Could it simply be a convenient way of covering up our ignorance of the complex dynamic neurobiology underlying the experience of pain? The American Academy of Pain Medicine attempted a similar sleight of hand when it coined the terms “eudynia” (good pain) and “maldynia” (bad pain).
I agree, that ‘maladaptive’ is to simplistic to explain e.g. chronic pain. However, in my mind I have what the H. Flor raised, the phenomenon occurring in the state which does not fulfill biological purposes…
This is a teleological argument.
What do you propose?
Dispense with the term “maladaptive neuroplasticity”.
Really like this piece of research. Very nice to know that we have a simple screening tool for a pain phenotype. I do wonder, however, if we have treatments that are specific enough to a particular phenotype to warrant use of the screening tool in clinical practice? Would love to hear people’s thoughts on this.
To jump from “pain descriptors” to “phenotype (as defined above)’ requires a huge conceptual leap of faith that is not justified. Incidentally, the IASP is currently exploring the case for a third pain descriptor to be used when neither nociception nor neuropathy can be demonstrated.